Eli Lilly likely exempt from Trump’s new pharmaceutical tariffs after $50B U.S. investment pledge
Eli Lilly’s $50 billion U.S. manufacturing expansion, including four new plants breaking ground in 2025, likely exempts the company from Trump’s new 100% pharmaceutical import tariffs.

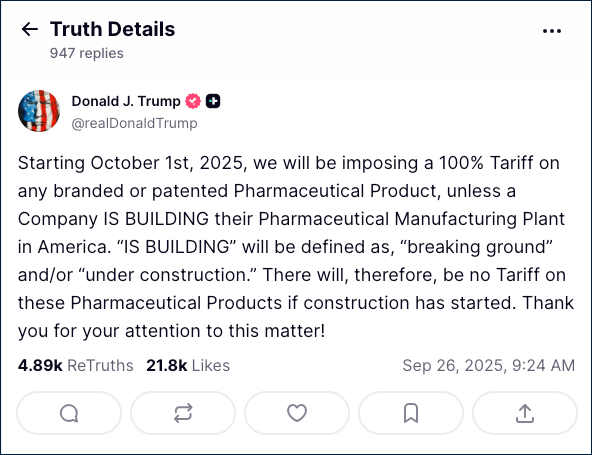
When President Donald Trump announced sweeping new import taxes this week—including a 100% tariff on pharmaceutical drugs beginning Oct. 1—he made one significant exception: companies currently building manufacturing plants in the United States. That carve-out appears to shield one of America’s largest drugmakers, Eli Lilly and Company, from the brunt of the new policy.
In a Truth Social post on Thursday, Trump declared that the pharmaceutical tariffs would not apply to companies “breaking ground” or already “under construction” on U.S. facilities. The president framed the move as necessary “for National Security and other reasons,” saying the goal was to push more medicine production back onshore.
Just months earlier, Eli Lilly unveiled plans that line up squarely with Trump’s exemption. On February 26, 2025, the Indiana-based firm announced it would more than double its U.S. manufacturing investment since 2020, exceeding $50 billion in capital commitments. That includes four new pharmaceutical manufacturing sites scheduled to begin construction this year, focused on active pharmaceutical ingredients and injectable therapies.
This marked the largest pharmaceutical manufacturing investment in U.S. history, and was followed most recently by an announcement that Ely Lily will build a $6.5 billion manufacturing facility at Generation Park in Houston, Texas. Lilly executives said the projects would create more than 3,000 highly skilled jobs and nearly 10,000 construction jobs, strengthening domestic supply chains and preparing for future demand across therapeutic areas like cardiometabolic health, oncology, immunology, and neuroscience.
"Lilly's optimism about the potential of our pipeline across therapeutic areas – cardiometabolic health, oncology, immunology and neuroscience – drives our unprecedented commitment to our domestic manufacturing build-out. Our confidence positions us to help reinvigorate domestic manufacturing, which will benefit hard-working American families and increase exports of medicines made in the U.S.A.."
-David A. Ricks, Lilly chair and CEO.
Trump’s tariff framework leaves much still undefined, with no formal Commerce Department guidance yet issued. But based on the president’s own wording, Lilly’s expansion should qualify. The company’s projects are clearly set to break ground in 2025, meeting the “under construction” threshold for exemption.
That would allow Lilly to continue importing needed inputs for its drug portfolio without facing sudden price spikes from the 100% tariff — a relief both for the company and potentially for U.S. patients who rely on its medicines.
While smaller pharmaceutical firms with no new U.S. buildouts may be hit with sharply higher costs, Lilly’s unprecedented investment appears to place it firmly on the safe side of the new trade barrier.